Help Relieve
the pressure
of hypertension
today
Under the latest guidelines, an estimated 45% of US adults are hypertensive.1,2,*

A Powerful ARB
Abbreviation: ARB, angiotensin II
receptor blocker

A Powerful ARB
+ Diuretic
Reduce SBP,
Reduce CV Risk5,6,†
Reduce CV Risk5,6,†
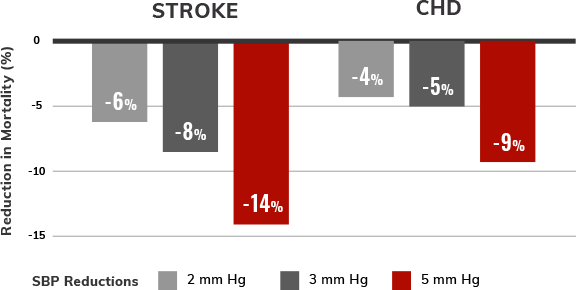
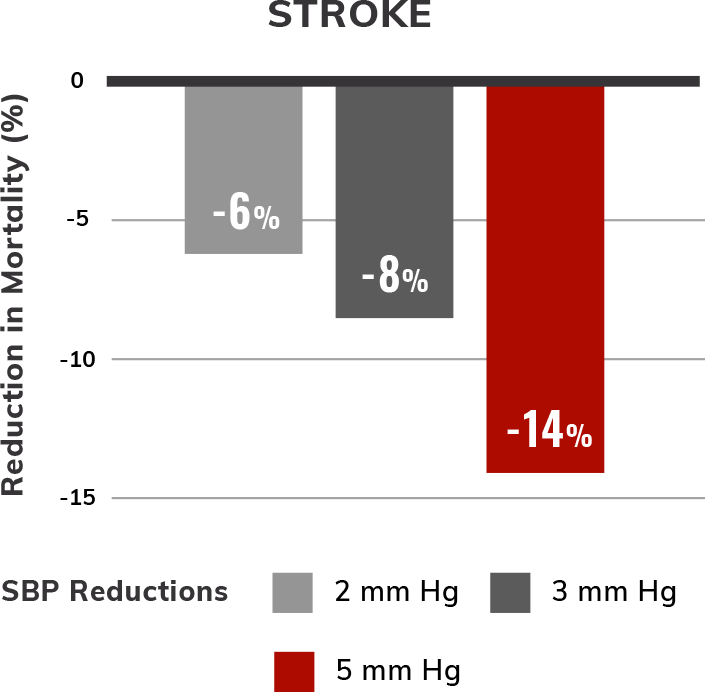
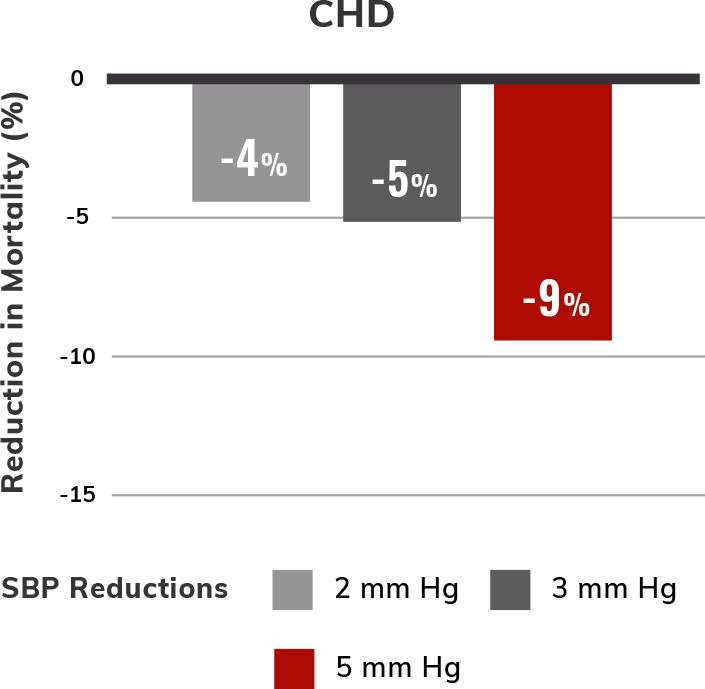
SBP treatment and control in patients with hypertension have been shown to reduce overall mortality, CV mortality, stroke, and heart failure (HF) events.5,6 Even 2 to 5 mm Hg SBP reductions may lower CV risk.6
†Study Design
SBP and mortality were analyzed in 5 large population follow-up studies: Multiple Risk Factor Intervention Trial, Whitehall Study, Chicago Western Electric Study, Framingham Heart Study, and Chicago Heart Association Detection Project in Industry. For each study, the association between initial SBP and death was examined. Follow-up lasted 6 to 19 years. Multivariate coefficients for these studies were similar and averaged.
CV RISK REDUCTION WITH EDARBI AND EDARBYCLOR HAS NOT BEEN ESTABLISHED.
Estimated reductions in CV mortality with modest lowering of SBP5,6,†,‡
Reduce SBP,
Reduce CV Risk5,6,†
Reduce CV Risk5,6,†
SBP treatment and control in patients with hypertension have been shown to reduce overall mortality, CV mortality, stroke, and heart failure (HF) events.5,6 Even 2 to 5 mm Hg SBP reductions may lower CV risk.6
Estimated reductions in CV mortality with modest lowering of SBP5,6,†,‡
†Study Design
SBP and mortality were analyzed in 5 large population follow-up studies: Multiple Risk Factor Intervention Trial, Whitehall Study, Chicago Western Electric Study, Framingham Heart Study, and Chicago Heart Association Detection Project in Industry. For each study, the association between initial SBP and death was examined. Follow-up lasted 6 to 19 years. Multivariate coefficients for these studies were similar and averaged.
CV RISK REDUCTION WITH EDARBI AND EDARBYCLOR HAS NOT BEEN ESTABLISHED.
Hear From Patients Who
Have Taken Control of Their
Blood Pressure (BP)
1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. doi:10.1161/CIR.0000000000000757
2. Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol. 2018;3(7):572-581. doi:10.1001/jamacardio.2018.1240
3. Edarbi [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC; [2020].
4. Edarbyclor [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC; [2020].
5. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252.
6. Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17(suppl 1):116-120.

